Which of the Following Best Describes a Double Replacement Reaction
See the answer See the answer done loading. Double replacement reaction O acid base reaction.

A Synthesis Reaction Is A Type Of Reaction In Which Multiple Reactants Combine To Form A Single Product Synthesis React Chemical Reactions Chemistry Synthesis
The overall pattern of a double replacement reaction looks like this.

. AB CD AD CB. Group of answer choices double replacement reaction acid base reaction neutralization redox reaction there is no reaction. What happens in a double-displacement reaction but does not happen in a combustion reaction.
Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds. Sodium carbonate decomposes to sodium oxide and carbon dioxide gas. Which of the following best describes the reaction if any that occurs when aqueous solutions of iron III nitrate and sodium iodide are combined.
Click here to view We have moved all content for this concept to for better organization. When the two reactants are mixed the potential products are Ag3PO4 and LiNO3. DESCRIPTION- the reaction is double displacement reactiondouble replacement reactionmetathesis double View the full answer Transcribed image text.
The positive hydrogen ion on the Chlorine has been replaced by a positive sodium ion on the Chlorine. This is a double replacement reaction that is also a neutralization It is a double replacement because the reaction starts with two compounds and ends with two compounds where the positive and negative ions have changed places. What term refers to a chemical reaction that absorbs heat energy.
Which of the following best describes the reaction if any that occurs when aqueous solutions of ironIII nitrate and sodium iodide are combined. Choose the general equation for the double replacement reaction. In other words it is where an element reacts with a compound and replaces one component of the compound.
Which of the following statements describes a double displacement reaction. Photosynthesis is not a single reaction pathway but two one dependent on the other. One element takes the place of another in a compound.
See answers 2 Best Answer. The the following reaction is. No it is a double replacement reaction.
We have a new and improved read on this topic. HC2H3O2aq NaOHaq NaC2H3O2aq H2Ol Aqueous solutions of acetic acid and sodium hydroxide react to produce aqueous sodium acetate and water. A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products.
2 When magnesium is burned in the presence of oxygen it produces magnesium oxide according to the following chemical equation. Click here to get an answer to your question which of the following best describes a double-replacement reaction. A single-replacement reaction also called a single-displacement reaction is one in which a pure element and a compound react chemically so that the products include another pure element and a different compound via exchanging of two species.
Please update your bookmarks accordingly. A precipitate of silver chloride forms when solutions of sodium chloride and silver nitrate are combined. Click Create Assignment to assign this modality to your LMS.
Srs H2Ol SrOH2aq H2g A decomposition reaction B neutralization reaction C single-replacement reaction D combination reaction E double-replacement reaction 29 Which of the following reactions is incorrectly classified. Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions. Therefore your answer is photosynthesis is a double replacement.
Sodium carbonate decomposes to sodium oxide and carbon dioxide. Which of the following describes a single-replacement reaction. Up to 24 cash back 28 What type of chemical reaction is illustrated in the following example.
Which of the statements below best describes the following reaction. Na2CO3s Na2Os CO2g A. Sodium loses electrons but chlorine gains electrons.
Which of the statements below best describes the following reaction. In the formation of sodium chloride by the combination of sodium and chlorine. Therefore a chemical reaction will occur and silver phosphate and lithium nitrate are the products.
Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas. According to rule 8 in Table 72 Ag3PO4 is insoluble meeting the requirements for a double-replacement reaction to happen. BaCO3 -- BaO CO2.
Which statement best describes. Describes the double-replacement reaction and gives examples.

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube

Chemistry Reactions Combination Reaction When Two Or More Reactions Combine To Form Chemical Reactions Chemical Reactions

Single Replacement Reaction Definition And Examples

Once You Have The Balanced Equation You Can Begin Putting Information Into The Bca Table Bca Stands For Before Change After You Start B Physique Chimie Chimie

Gait And Cog Vertical Direction Supportive Physics

Combustion Reactions Chemical Reactions Chemical Equation Reactions

A Mole Ratio Is The Ratio Amounts Of The Entities In A Chemical Reaction Chemistry Worksheets Chemistry Lessons Teaching Chemistry
What Are The Differences Between Single And Double Displacement Reactions Quora
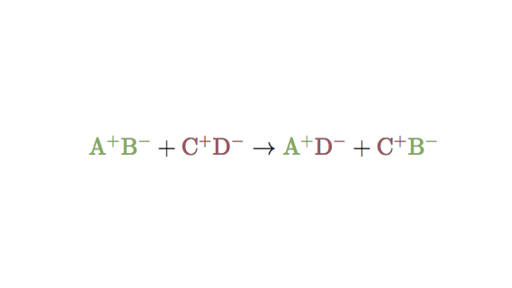
Double Replacement Reactions Double Displacement Article Khan Academy

Plus One Chemistry Notes Chapter 2 Structure Of Atom A Plus Topper Chemistry Notes Chemistry Atomic Structure

Strontium Chloride In Burning Methanol Holymoleculesbatman Science Technology Engineering Art Math Natural Landmarks Science And Technology

Exothermic Reactions Release Energy Endothermic Reactions Consume Energy Exothermic Reaction Homeschool Science Chemistry

Image Result For Ordinal Vs Nominal Learning Mathematics Data Sigma

Chemical Formula Writing Simplified Writing Worksheets Chemical Formula Writing

Predicting Products For Decomposition Reactions Youtube Reactions Mo Co Chemistry
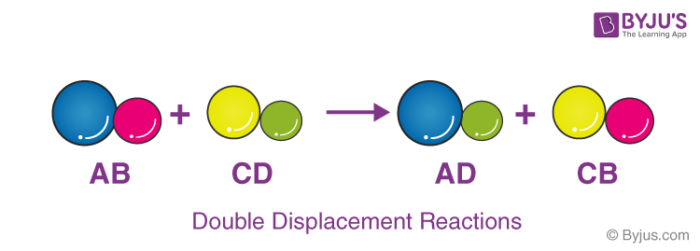
What Is Double Displacement Reaction Chemistry Question
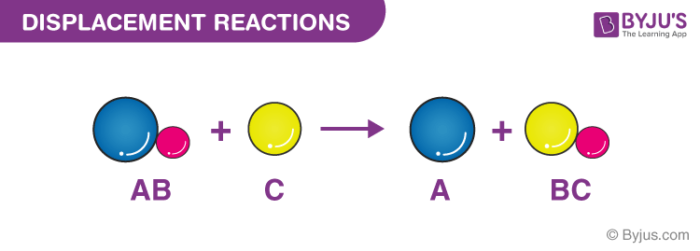
Displacement Reactions Definition Types Single Double Examples

Comments
Post a Comment